Baltic Sea
The Baltic Sea was already during the 1970s considered as one of the most polluted seas in the world (Elmgren 1989; Gänzle 2011). The environmental deterioration during the 20th century is clearly linked to collective action problems among different user groups such as farmers, fisheries and industry. Farming practices has generated substantial pollution of nutrients in the Baltic Sea, which has resulted in large-scale increases in primary production, but also in hypoxia in the deep layers of the offshore areas. The institutional arrangements for addressing such deterioration of common resources has not proven efficient, despite the fact that HELCOM, the international forum for environmental cooperation, has been in operation for more than 30 years. One reason is the diffuse sources of pollution that are generated from farming practices, but also the fact that nutrient pollution is also derived from e.g. transport, the forestry sector and individual households. HELCOM has, on the other hand been relatively successful in reducing toxic pollutants, by organizing a program for identification and mitigation of polluting “hot spots”. The “hot spots” program represents a way to effectively organize collective action to address pollution, primarily resulting from industry. This “end-of-pipe” type of pollution has been easier to address than the diffuse pollution resulting from nutrients, from a diversity of sources in multiple sectors.
Fisheries represent another collective action problem where a large number of individuals from different countries harvest shared resources. Despite a developed system of quota allocations for some species between countries, it is clear that problems associated with monitoring, enforcement and compliance represent important collective actions problems. A number of fish stocks that are unregulated, or the capture of other species (e.g. seabirds and seals) represent other collective action problems in the Baltic Sea environment.
The Baltic sea ecosystem
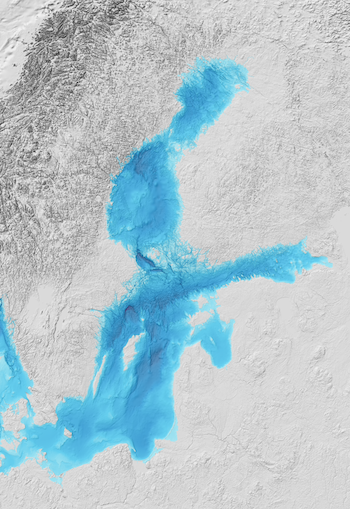
The Baltic Sea is the largest stable brackish sea in the world. Its very narrow connection to the open ocean and large rivers that feed into it results in a stable salinity gradient. One important consequence of this rare environmental condition is that species richness in the main lower basin is remarkably low, as for both ocean living species and freshwater species it is their tolerance levels of salinity. The Baltic Sea has one of the lowest species numbers in the world. But due to its high inflow of freshwater with nutrients from land sources, it is also one of the most productive, for the good and bad.
Substantial ecological dynamics have been observed in the Baltic Sea during the last century, including regime shifts. A first ecological regime shifts was a shift from an oligotroph state to a eutrophic state in the mid 1900s, caused by anthropogenic nutrient loading and associated negative feedback mechanisms. An increased primary production resulting from increases in nutrient loading resulted in an increase in decomposing biological material in the deep layers of the Baltic Sea, and an increased consumption of oxygen from the material that decomposes in the sediments. Phosphorous from this material is bound in the sediment as long as they are oxygenated, but with an increased level of anoxia, phosphorous is re-released, which in turn stimulated further plankton productivity reinforcing this regime. The regime shift is identified in 1950, simultaneous to a rapid increase in the extent and duration of anoxia only to be occasionally replenished with oxygen and salty water when weather conditions caused large inflow of ocean water through the sound between Denmark and Sweden. Due to concerted efforts of monitoring and data collection [Baltic Nest][http://www.balticnest.org] and a multinational commission, the Baltic Marine Environment Protection Commission HELCOM, the eutrophication has past its peak in 1981 and is expected to continue to decline as adjacent nations coordinate their efforts.
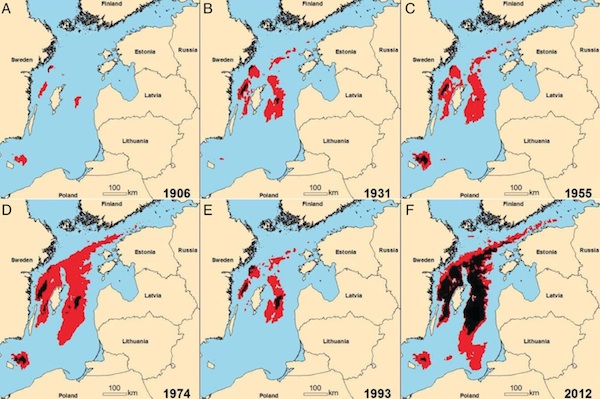
In the 1980’s, fish production, such as cod, was at its peak. A second shift occurred from a cod dominated state to a clupeid dominated state in the late 1980s, which has been described as resulting from overfishing of cod, climate-driven changes in zooplankton composition and deteriorating conditions for cod reproduction, including an increasing extent of anoxic zones where cod are unable to reproduce (Casini et al. 2008; Möllmann and Köster 1999; Österblom et al. 2007). When cod are abundant they may control the population of sprat which releases the predation on the main copepod which is in turn important for cod larvae survival and growth. Thus, many adult cod improve the conditions for cod recruitment, constituting one feedback loop. If cod abundance is reduced, as occurred due to low oxygen and low saline environment during the 1988-1993 as well as through unsustainable fishing rates, sprat can increase in abundance. Sprat not only compete with cod larvae for food, but also directly feed on cod larvae, thus constituting another competing feedback loop. As the environmental conditions improved after 1993, the food web remained locked into the second feedback-loop. From a fisheries economics perspective, this was a disaster, as the same system potentially released into the first regime would constitute far higher revenue for the Baltic sea fisheries.
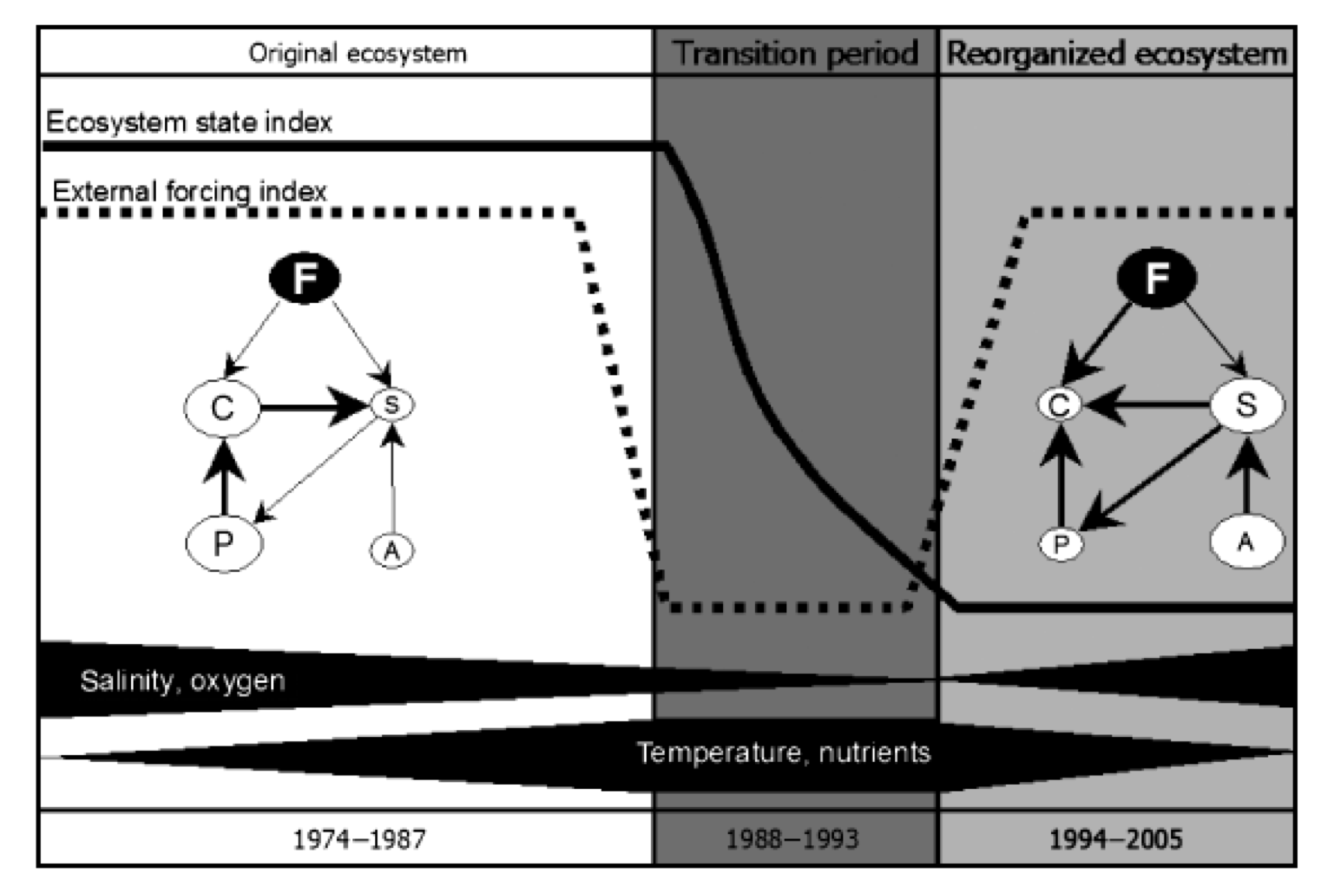
These shifts still pose difficult challenges for the governing system as they are difficult to reverse. Political decisions in HELCOM has improved the prospects for dealing with nutrient pollution, but measures are not likely to have an effect for at least a decade, dependent on systems inertia and ecological feedbacks. Making a eutrophic regime relatively resilient. Measures directed at restoring the cod stock are hampered by additional complications, as the cod stock is currently suffering from very low levels of growth.
Continuing but also new anthropogenic pressures pose new challenges, such as increased shipping, and wind, wave and gas pipelines in and through the Baltic Sea. An additional challenge is that of introduced species, with unclear ecological effects. All these challenges come with great uncertainties making the governing system extra vulnerable for quick environmental changes in the years to come.
The Baltic Sea multilevel governance
The challenge to overcome a type of tragedy of the commons situation in the Baltic Sea has been ongoing in the region, at several management levels since the first United Nations Conference on the Human Environment 1972 (also known as the Stockholm Conference). Only two years after the Stockholm Conference the intergovernmental collaboration that the Helsinki Commission started. Their goal is for the nation states to jointly govern and improve the environmental state of the Baltic Sea. This has primarily been done by creating a common vision and understanding of a healthier Baltic Sea (Valman 2013). Besides the nation states and the EU are several stakeholders involved in information sharing and knowledge production within the Helsinki Commission (Valman et al. 2014; VanDeveer 2011). Several other organizations and collaborations within the Baltic Sea region also exist forming an overlapping and nested system of actors. The Helsinki Commission first and foremost deal with different kinds of pollutants, where nutrients causing eutrophication is counted as one. To push for further improvements of the Baltic marine environment, the Helsinki Commission launched the Baltic Sea Action Plan in 2007, where the ecosystem approach to management is a cornerstone.
An important feature of the ecosystem approach is that the management processes should be adaptive. At the international level, the adaptiveness of the Baltic Sea governance could be improved in several ways. As of now adaptiveness and flexibility lies and are steered at the respective state levels in the Baltic Sea (Valman et al. 2014).
Management of fisheries lies beyond the mandate of the Helsinki Commission. Instead, decisions for quotas are taken in annual meetings with the European Council of Ministers. Individual member states have relatively large freedom of regulating and monitoring their own fisheries within the internationally agreed systems of quotas. In the Baltic Sea, systematic misreporting of species composition have led to that the management have underestimated the total catches of fish in the Baltic Sea, leading to an overcapacity in the fishery (Hentati-Sundberg et al. 2014). Many of the large scale commercial fisheries do not represent a self-organizing systems, rather they are driven by top-down management regulations, which has favored increasing scale of fishery operations and specialization (Boonstra and Hentati-Sundberg 2014). There are, however, a number of small-scale fisheries in operation with substantial ability to self-organize their own context, and such co-management initiatives have been stimulated in some countries.
Besides the international governance of the Baltic Sea there are also management efforts going on at more regional and local levels in the region. Co-management initiatives are, for example, taking place in coastal communities in Sweden for implementing the ecosystem approach (Sandström et al. 2014). Co-management of fisheries has also been explored, steered by the Swedish National Board of Fisheries (Fiskerieverket 2007).
Contributors
Henrik Österblom, Matilda Valman, Thorsten Blenckner, Jon Norberg
References
Fiskeriverket (2007), ‘Regional och lokal samförvaltning av fiske’, Dnr 239-457-04. Also available at: https://www.havochvatten.se/download/18.64f5b3211343cffddb2800022876/1348912826299/samforvaltning-av-fiske.pdf
Boonstra, Wiebren J. and Hentati-Sundberg, Jonas (2014), ‘Classifying fishers’ behaviour. An invitation to fishing styles’, Fish and Fisheries:n/a-n/a.
Casini, Michele, Lövgren, Johan, Hjelm, Joakim, Cardinale, Massimiliano, Molinero, Juan-Carlos, and Kornilovs, Georgs (2008), ‘Multi-level trophic cascades in a heavily exploited open marine ecosystem’, Proceedings of the Royal Society B: Biological Sciences, 275(1644):1793-1801.
Elmgren, Ragnar (1989), ‘Man’s Impact on the Ecosystem of the Baltic Sea: Energy Flows Today and at the Turn of the Century’, Ambio, 18(6):326-332.
Gänzle, Stefan (2011), ‘Introduction: Transnational Governance and Policy-Making in the Baltic Sea Region’, Journal of Baltic Studies, 42(1):1-7.
Hentati-Sundberg, J., Hjelm, J., and Österblom, H. (2014), ‘Does fisheries management incentivize non-compliance? Estimated misreporting in the Swedish Baltic Sea pelagic fishery based on commercial fishing effort’, ICES Journal of Marine Science: Journal du Conseil. Möllmann, Christian and Köster, Friedrich W. (1999), ‘Food consumption by clupeids in the central Baltic Sea: evidence for top-down control?’, ICES Journal of Marine Science, 56:100-113.
Sandström, Annica, Crona, Beatrice, and Bodin, Örjan (2014), ‘Legitimacy in Co-Management: The Impact of Preexisting Structures, Social Networks and Governance Strategies’, Environmental Policy and Governance, 24(1):60-76.
Valman, Matilda (2013), ‘Institutional stability and change in the Baltic Sea: 30 years of issues, crises and solutions’, Marine Policy, 38:54-64.
Valman, Matilda, Österblom, Henrik, and Olsson, Per (2014), ‘Adaptive governance of the Baltic Sea: Lessons from elsewhere’, International Journal of the Commons, (In press). VanDeveer, Stacy D. (2011), ‘Networked Baltic Environmental Cooperation’, Journal of Baltic Studies, 42(1):37-55.
Österblom, Henrik, Hansson, Sture, Larsson, Ulf, Hjerne, Olle, Wulff, Fredrik, Elmgren, Ragnar, and Folke, Carl (2007), ‘Human-induced Trophic Cascades and Ecological Regime Shifts in the Baltic Sea’, Ecosystems, (10):877-889.
Leave a Comment